Lack of physical activity and sleep are two key risk factors for numerous physical and psychological health-related issues (Colten and Altevogt, 2006; Center for Disease Control (CDC), 2015). Lack of physical activity is linked to an increased risk of physical health issues, such as cardiovascular disease and type 2 diabetes; mental health issues, including depression and anxiety; and cognitive issues, such as deficiencies in learning and judgement (Guszkowska, 2004; CDC, 2015). Lack of sleep duration is also associated with numerous chronic diseases, including hypertension, diabetes, depression, obesity, and cancer, along with increased mortality, reduced quality of life, and impaired cognitive function (Colten and Altevogt, 2006).
One population likely to lack physical activity and sleep is postpartum mothers. Postpartum mothers are less active than women of the same age without children (Verhoef et al, 1993; Marcus et al, 1994; Drago, 2001) and mothers with older children (Marcus et al, 1994; Brown and Trost, 2003). Furthermore, postpartum mothers have reported getting 1–2 fewer hours of sleep than their non-postpartum counterparts (Quillin, 1997; Thomas and Foreman, 2005). Along with negative consequences for the mother, lack of physical activity and sleep in postpartum mothers may have implications for their child, such as reduced alertness and decision-making (Harrison and Horne, 2000). Additionally, lack of physical activity and sleep have both been linked to an increased risk of depression in mothers, which is associated with adverse cognitive and emotional development in their children (Beck, 1998; DØrheim et al, 2009; Teychenne et al, 2010). Specifically, maternal depressive symptoms at 6 months postpartum have been associated with lower vocabulary scores and higher rates of behavioral issues in children at age 5 years (Brennan et al, 2000).
Similar to lack of physical activity and sleep, carrying excessive body weight may lead to adverse effects in postpartum mothers and their children. Postpartum mothers who are also overweight/obese may be more likely to experience unfavourable health outcomes than normal-weight mothers. Carrying excessive body weight increases the risk of heart disease, cardiovascular disease, type 2 diabetes, certain cancers, osteoarthritis and depression (Dixon, 2010; CDC, 2015). Along with the aforementioned developmental risks associated with maternal depression, having an overweight/obese mother also increases the risk for obesity in the child, placing the current and future health of the child at risk (Beck, 1998; Brennan et al, 2003; Reilly et al, 2003; Gibson et al, 2007). Because of the relationship between the health of a mother and her child, it is critical that research continues to explore this topic.
While it is known that physical activity and sleep are negatively impacted during the postpartum period, little research has examined if these risks are compounded for overweight/obese mothers. Existing research has primarily relied on self-reported measures such as weekly recalls or diaries, and/or collected data with a one-time assessment (Wolfson et al, 2003; Wilkinson et al, 2004; Gunderson et al, 2008; Montgomery-Downs et al, 2010). Research examining physical activity and sleep trends in mothers, especially those who are also overweight/obese, is therefore needed to provide empirical evidence and potentially serve as a foundation for future research and interventions. This study aimed to increase understanding of physical activity and sleep trends in postpartum mothers by examining trends in postpartum mothers' physical activity and sleep between 3–6 months postpartum, and comparing physical activity and sleep between overweight/obese and normal-weight postpartum mothers. ‘Normal weight’ was defined as a body mass index (BMI) of 18.5–24.9 kg/m2, and ‘overweight/obese’ was defined as a BMI of >25 kg/m2.
Methods
Participants
Participants were recruited as part of a larger study examining the relationship between physical activity and motor skill development in infants in normal and overweight infants. To determine the level and variability of physical activity, infants and mothers wore accelerometers for 4 consecutive days at three different time points: initial appointment (child mean age= 3 months, 3 days ± 11.98 days), at the onset of their child's sitting (child mean age=5 months, 3 days ± 27.15 days), and 1 month after infant's onset of sitting (child mean age=6 months, 5 days ± 26.24 days). Mothers' accelerometer data were used for the present study.
Mothers with an infant aged less than 3 months old were recruited from family-friendly businesses such as recreation and daycare centres; medical centres such as doctors' offices; and women, infants and children clinics (a public health nutrition programme for low-income families) in Omaha, Nebraska, a Midwestern city in the USA; as well as via internet sources such as social media and baby-friendly websites. Flyers were distributed at these locations with a brief description of the study and, if interested, potential participants could contact the second author via email or telephone. A more detailed description of the study was given and if mothers were interested, a time was arranged for data collection. Although mothers were recruited when their infant was less than 3 months old, they were not included in the study until their infant had reached 3 months of age. Mothers were included if they were at least 19 years of age, and had an infant at least 3 months old. Participants provided written consent to participate, and received $35.00 (£26.00) for completing the study.
Of the 51 mothers who stated an interest in participating, 21 completed the study. The majority (n=15) of those who withdrew did so before the first visit. The other 15 who were not included either withdrew after the first visit (n=6), missed one of the three visits (n=5), or did not wear the accelerometer for an extended period of time (n=4).
Ethical approval
All procedures complied with legal and institutional guidelines. Approval was received from the University of Nebraska Medical Center Institutional Review Board before the study began.
Procedures
Mother and infants visited a university motor development laboratory at each time point: initial appointment (3 months of age), onset of their child sitting, and 1 month after onset of sitting. At the first visit, demographic information, including race/ethnicity, age, education level, and income, were obtained. Mothers' anthropometry (height and weight) was assessed at each of visit via a self-report survey. BMI was calculated using height and weight measurements from the third visit.
Physical activity and sleep were measured by determining daily moderate-to-vigorous physical activity and sleep, in minutes. Physical activity and sleep data were gathered via an accelerometer device, the Actigraph GT9X Link, worn on the non-dominant wrist. The Actigraph GT9X Link is a lightweight, water-resistant model that can record and store raw acceleration data at a frequency ranging from 30-100 Hertz. Frequency and intensity are recorded as counts/minute. The accelerometers were worn for 4 consecutive days (2 weekdays, 2 weekend days) after each of the three visits. Mothers were asked to wear the accelerometers at all times (excluding bathing or swimming, as the device has limited submersion protection). If a participant was unable to wear the devices for an extended period of time (>30 minutes), they were asked to report this time to the researchers. This report helped to determine if there were gaps in wear time. Only participants who wore the device for 4 consecutive days without extended periods of non-wear time were included in data-analysis. To minimise the amount of non-wear time, participants were sent a reminder e-mail or text the first night of expected wear and then a follow-up text 2 days into the data collection.
Accelerometer
In terms of physical activity, wrist-worn Actigraph accelerometers have proven accurate for use through validation against indirect calorimetry (Swartz et al, 2000; Ekelund et al, 2001; Hildebrand et al, 2014). GGIR version 1.3-2 with R-package (Van Hees et al, 2014) was used to evaluate the physical activity data. The R-package can clean accelerometer data, detect non-wear periods, and extract the user-defined acceleration levels, which classified the physical activity intensity levels (MET values) (Van Hees et al, 2014). Hildebrand's intensity cut points were used to classify physical activity level (Hildebrand et al, 2014). Moderate-intensity physical activity threshold was 100.6 mg and vigorous-intensity physical activity threshold was 428.8 mg expressed in gravity-based acceleration units (g).
Wrist-worn Actigraph accelerometers have also been validated as an indirect assessment of sleep and are strongly correlated to polysomnography, the gold-standard of sleep assessment (de Souza et al, 2003; McCall and McCall, 2012; Kosmadopoulos et al, 2014). Wrist-worn accelerometers are recommended as a sleep measure, as they are more objective than self-report measures and do not require the invasiveness of polysomnography, which can lead to sleep disturbances (Morgenthaler et al, 2007). The Tudor-Locke algorithm was used to assess sleep data (Tudor-Locke et al, 2014). This algorithm was chosen because it combines Sadeh's sleep algorithm and inclinometer results to classify time-sampling intervals or epochs (Butkov and Lee-Choing, 2007).
Statistical analysis
All statistical analyses were performed using SPSS version 23. The limit for statistical significance was set at P<0.05. Mean and standard deviations were calculated for physical activity and total sleep time. The results for model assumptions of normality, homogeneity of covariance, and linearity were checked. Effect size (Cohen's d) was calculated to display the magnitude of differences over time and between groups. Effect size calculations provide additional context when comparing means, allowing researchers to determine the size of the difference, as opposed to merely whether differences were statistically significant or not (Sullivan and Feinn, 2012). A larger effect size indicates a larger magnitude of difference between means. Effect size was classified as trivial (d≤0.2), small (d=0.2–0.49), moderate (d=0.5–0.79), or large (d≥0.8) (Cohen, 1988). Data were analysed and reported in two ways. First, combined data of all participants—overweight and normal weight—were analysed collectively. Data were also split into categories based on weight status—overweight and normal weight—and comparisons between groups were made. A two-way repeated measures ANOVA was conducted to determine changes in mean values over time while comparing overweight and normal weight groups.
Results
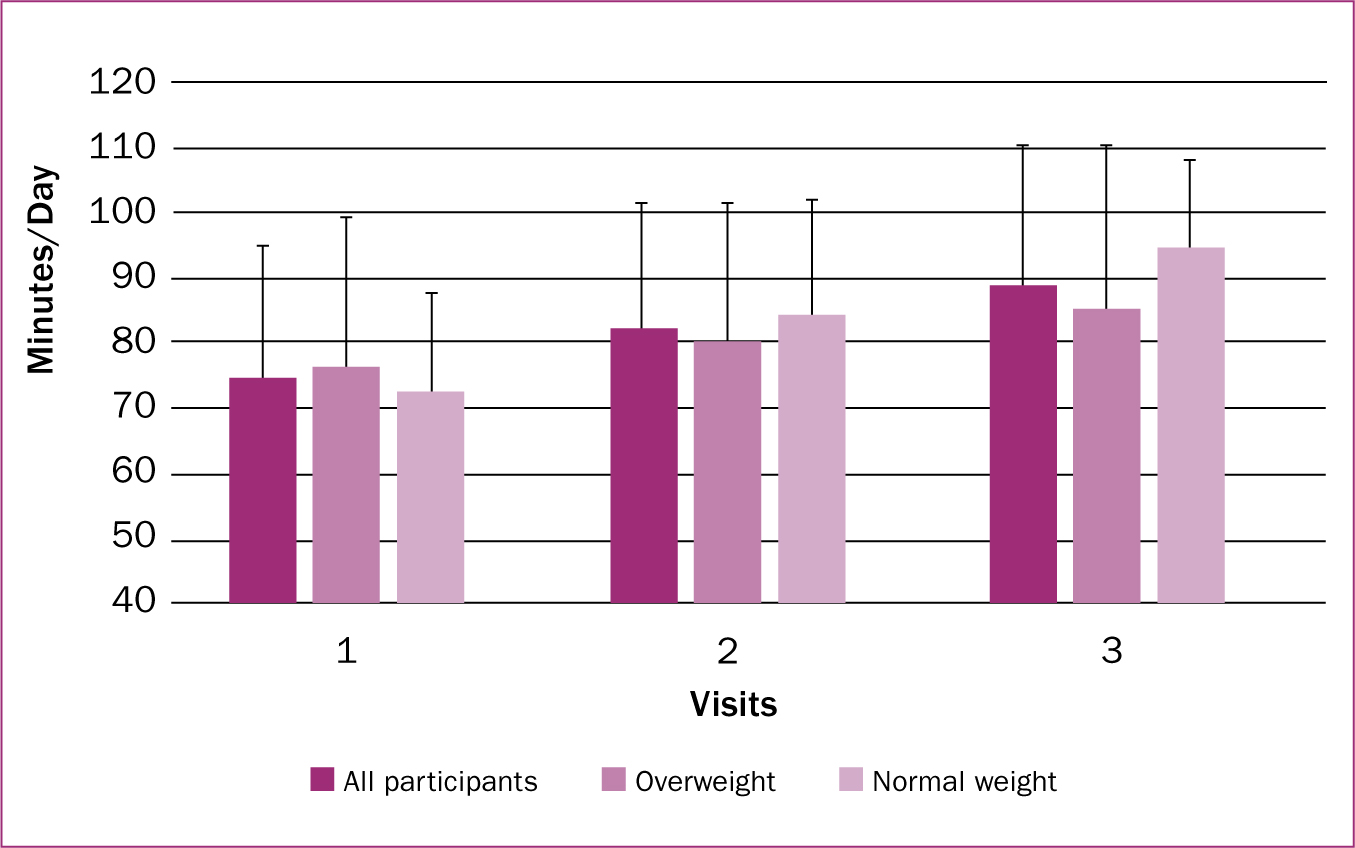
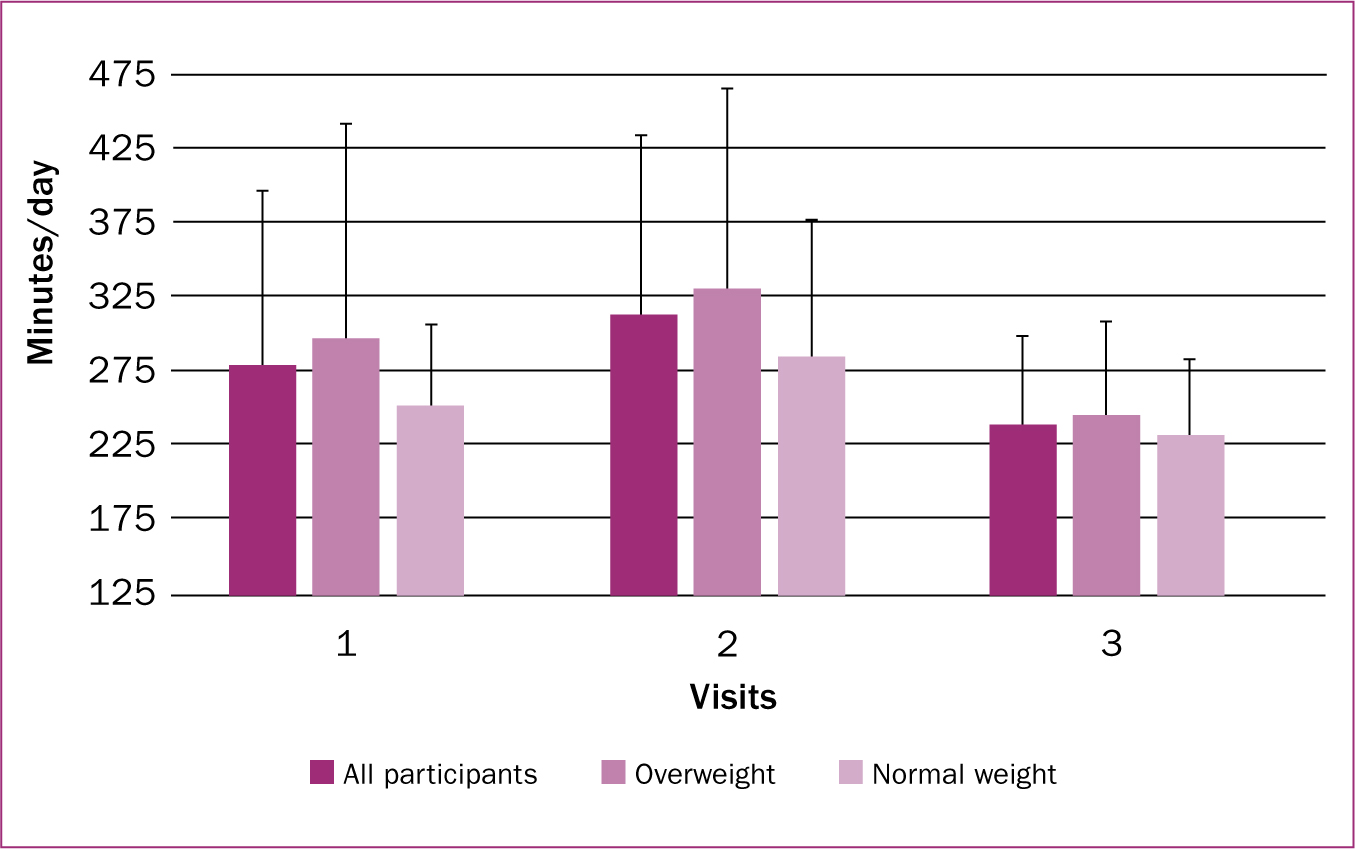
All participants had a mean BMI of 27.9 ± 6.5 kg/m2 and average age of 30.4 ± 3.2 years. Participants in the overweight/obese group (n=13) had an average BMI of 31.96 ± 5.0 kg/m2 and average age of 29.38 ± 3.1 years, while participants in the normal weight group (n=8) had an average BMI of 21.53 ± 1.4 kg/m2 and average age of 32.12 ± 2.6 years. No participants switched weight classifications over the course of the study. Additional participant characteristics are described in Table 1. Means and standard deviations for physical activity and sleep by visit and weight classification are provided in Figures 1 and 2, respectively.
| Total (n=21) | Normal weight (n=8) | Overweight/obese (n=13) | |
|---|---|---|---|
| Characteristic | n (%) | n (%) | n (%) |
| Age (years) | |||
| 20–30 | 13 (61.9) | 3 (37.5) | 9 (69.2) |
| 31–40 | 8 (38.1) | 5 (62.5) | 4 (30.8) |
| Mean (SD) | 30.4 (±3.2) | 32.12 (±2.6) | 29.38 (±3.1) |
| Body mass index (BMI) (kg/m2) | |||
| 18.5–24.9 | 8 (38.1) | 8 (100.0) | 0 (0.0) |
| 25–29.9 | 6 (28.6) | 0 (0.0) | 6 (46.2) |
| >30 | 7 (33.3) | 0 (0.0) | 7 (53.8) |
| Mean (SD) | 27.9 (±6.5) | 21.53 (±1.4) | 31.96 (±5.0) |
| Race | |||
| White | 19 (90.5) | 7 (87.5) | 12 (92.3) |
| Other | 2 (9.5) | 1 (12.5) | 1 (7.7) |
| Highest education | |||
| Secondary | 2 (9.5) | 1 (12.5) | 1 (7.6) |
| Some college | 6 (28.6) | 0 (0.0) | 6 (46.2) |
| Undergraduate degree | 6 (28.6) | 3 (37.5) | 3 (23.1) |
| Graduate degree | 7 (33.3) | 4 (50.0) | 3 (23.1) |
| Employment | |||
| Full-time | 9 (42.9) | 4 (50.0) | 5 (38.5) |
| Homemaker | 9 (42.9) | 3 (37.5) | 6 (46.2) |
| Other | 3 (14.2) | 1 (12.5) | 2 (15.4) |
| Household Income | |||
| $21k–$40k (£15k–£30k) | 3 (14.2) | 0 (0.0) | 3 (23.1) |
| $41k–$60k (£31k–£45k) | 2 (9.5) | 1 (12.5) | 1 (7.6) |
| $61k–$80k (£46k–£60k) | 12 (57.1) | 5 (62.5) | 7 (53.8) |
| $81k+ (£61k+) | 4 (19.0) | 2 (25.0) | 2 (15.4) |
Physical activity
All participants
There was a significant effect in physical activity levels of all mothers (F (2, 18) = 5.56; P=0.013). There was a non-significant increase with a trivial effect in physical activity between visits one and two (difference=8.26 min/day; P=0.161; d=0.185), a non-significant increase with a moderate effect in physical activity levels between visits two and three (difference=7.46 min/day; P=0.639; d=0.165), and a significant increase with a moderate effect in physical activity levels between visits one and three (difference=15.72 min/day; P=0.018; d=0.325).
Normal weight and overweight/obese
There was no interaction effect between moderate-to-vigorous physical activity and weight classification (F (2, 18)=0.965; P=0.40). However, there was a significant effect in moderate-to-vigorous physical activity in the normal weight group (F (2, 18)=4.50; P=0.026). There was a non-significant increase with a small effect between visits one and two (difference=11.94 min/day; P=0.222; d=0.345), a non-significant increase with a large effect between visits two and three (difference=10.14 min/day; P=0.838; d=0.313), and a significant increase with a large effect between visits one and three (difference=22.08 min/day; P=0.037; d=616).
In the overweight/obese group, no significant main effect on physical activity was found (F (2, 18)=1.25; P=0.311). There was a non-significant increase with a small effect between visits one and two (difference=4.57 min/day; P=1.00; d=0.104), a non-significant increase with a small effect between visit two and three (difference=4.77 min/day; P=1.00; d=0.103), and a non-significant increase with a moderate effect in physical activity levels between visits one and three (difference=9.35 min/day; P=0.455; d=0.191).
Sleep
All participants
There was a significant effect on sleep levels of all mothers (F (1.97, 37.39)=3.610; P=0.037). There was a non-significant increase with a small effect between visits one and two (difference=33.6 min/day; P=0.241; d=0.280), a significant decrease with a moderate effect between visits two and three (difference=74.3 min/day; P=0.023; d=0.781), and a non-significant decrease with a small effect between visits one and three (difference=40.7 min/day; P=0.208; d=0.432).
Normal weight and overweight/obese
There was no interaction effect between sleep and weight classification (F (1.974, 38)=0.239; P=0.786). There was no significant effect in the normal weight group (F (1.511, 10.580)=1.438; P=0.273). There was a non-significant increase with a small effect between visits one and two (difference=44.1 min/day; P=0.434; d=0.462), a non-significant decrease with a moderate effect between visits two and three (difference=53.6; P=0.246; d=0.716), and a non-significant decrease with a small effect between visits one and three (difference=18.4 min/day; P=0.707; d=0.338).
In the overweight/obese group, there was no significant effect on sleep (F (1.896, 22.75)=2.317; P=0.124). There was a non-significant increase with a small effect between visits one and two (difference=32.64 min/day; P=0.429; d=0.233), a significant decrease with a large effect between visits two and three (difference=87.1 min/day; P=0.023; d=0.816), and a non-significant decrease with a small effect between visits one and three (difference=37.9 min/day; P=0.167; d=0.486).
Discussion
This study aimed to increase understanding of physical activity and sleep in postpartum mothers by examining trends in postpartum mothers' physical activity and sleep at three time intervals between 3–6 months postpartum, and comparing physical activity and sleep of overweight/obese and normal-weight postpartum mothers. Findings indicate that physical activity levels tended to increase over time in all mothers, although this increase was only significant in normal-weight mothers. Sleep levels increased at the second visit and decreased at the third. No interactions with weight classifications were found in either physical activity or sleep. The patterns observed in this study displayed trends in key health behaviours at three time points between 3–6 months postpartum.
Overall, the findings related to increasing physical activity levels added to an area in the literature that was previously lacking. It is known that physical activity levels tend to decrease during pregnancy; however, little is known about physical activity throughout the postpartum period (Borodulin et al, 2008). This study therefore suggests that postpartum mothers incrementally increase physical activity levels over time. These findings align with other studies suggesting that physical activity increases over the course of the first year postpartum (Treuth et al, 2005; Borodulin et al, 2009). On further examination, a difference was noted between the physical activity trends of normal-weight and overweight/obese participants. In normal-weight participants, physical activity levels increased (+20.9 min/day) from the first visit to the third visit, while physical activity levels increased with a small effect (+12.2 min/day) in overweight/obese mothers over the same interval.
Direct comparison of activity levels to similar studies is challenging because this study used wrist-worn accelerometers, which tend to overestimate physical activity, while others (Hildebrand et al, 2014) used waist-worn accelerometers. Because comparison of absolute physical activity values is limited, relative physical activity levels and trends were compared to previous research. Similar findings existed with this type of comparison (Davis et al, 2006; Tudor-Locke et al, 2010; Durham et al, 2011). In a study with postpartum mothers (Treuth et al, 2005; Borodulin et al, 2009), a high proportion of overweight/obese mothers maintained insufficient levels of physical activity based on the NHS's recommendations of 150 minutes of moderate intensity or 75 minutes of vigorous intensity physical activity per week (NHS Choices, 2015). Additionally, in adult non-pregnant/postpartum populations, being overweight/obese is associated with lower levels of physical activity (Davis et al, 2006; Tudor-Locke et al, 2010). For instance, Davis et al (2006) concluded that normal weight individuals achieved 21 minutes more moderate-to-vigorous physical activity each day than their overweight/obese counterparts. This study therefore illustrates a time period where efforts to increase physical activity may need to focus. The differences in degree of physical activity level increase between groups over time indicates that 5–6 months postpartum may be a critical period. Efforts aimed at preparing postpartum mothers to increase physical activity during this time are needed.
Due to differences in effect sizes and significance of physical activity over time between normal-weight and overweight/obese groups, it is important to investigate factors influencing these trends. First, physical activity during pregnancy is associated with postpartum physical activity (Pereia et al, 2007). Insufficient physical activity during pregnancy has been associated with an increased likelihood of insufficient activity at 6 months postpartum (Pereia et al, 2007). Thus, it is plausible that overweight/obese participants were previously less active than normal weight participants, similar to non-postpartum populations (Davis et al, 2006; Tudor-Locke et al, 2010). Second, key demographic differences between participants existed, specifically, related to income. Although the majority of both groups reported household incomes above $41 000 (£30 971), three participants in the overweight/obese group reported a household income of less than $40 000 (£30 209), while no normal-weight participants fell into this classification. In a study examining the correlates of postpartum physical activity, a low income was associated with lower moderate-to-vigorous physical activity (Vladutiu et al, 2015); therefore, it is important for future efforts aiming to increase physical activity in postpartum mothers to identify ways to help women with these characteristics overcome potential deterrents to physical activity.
One finding in this study was the participants' overall lack of sleep. When compared to previous studies measuring sleep in postpartum mothers (Wolfson et al, 2003; Wilkinson et al, 2004; Montgomery-Downs et al, 2010), participants in the present study achieved far less sleep. For instance, in a study examining changes over the first year postpartum (Wolfson et al, 2003), participants reported 434 minutes of sleep per day at 12-16 weeks postpartum. In a similar study (Wilkinson et al, 2004), participants averaged 502 minutes of sleep per day at 3 months postpartum. At visit one (3 months postpartum), participants averaged 278.94 minutes of sleep per day. This is important to consider, as lack of sleep is associated with postpartum weight retention and increased risk of depression (Xiao et al, 2014). Specifically, sleeping less than 5 hours per day at 6 months postpartum is associated with retaining ≥5kg of weight at 1 year postpartum (Gunderson et al, 2008). In a review by Bhati and Richards (2015), it was concluded that a relationship existed between sleep disturbances and postpartum depression, suggesting that efforts are needed to improve the sleep levels of postpartum mothers. Interestingly, participants sleep levels decreased from visit two to three. Due to data collection being 4 days and the small sample, it is possible that a short stretch of poor sleep was gathered.

Implications
Based on the lesser increase in physical activity of overweight/obese mothers, efforts to increase physical activity levels for overweight/obese postpartum mothers may be needed. To accomplish this, it is necessary to understand factors influencing physical activity in postpartum mothers. A mixed-methods approach aimed at understanding physical activity practices and barriers in overweight/obese postpartum mothers is therefore recommended. Additionally, because weight retention is associated with reduced postpartum physical activity levels, efforts including other lifestyle modifications that facilitate improvements in body composition—such as improvements in nutrition and sleep—may be effective in improving physical activity levels. In terms of sleep, efforts are needed to better understand sleep levels in postpartum mothers overall as well as improve wrist-worn accelerometers as a data collection tool for sleep. Efforts may focus on improving sleep education, as maternal and infant sleep levels have been shown to improve through a 5-week behavioural-educational intervention (Stremler et al, 2006).
Physical activity interventions during pregnancy and postpartum may address several issues encountered by participants in this study. Physical activity during pregnancy is associated with healthy gestational weight gain and increased postpartum physical activity, both of which influence physical activity directly as well as indirectly by improving postpartum weight retention (Öhlin and Rossner, 1994; Pereira et al, 2007; Nehring, et al, 2011). Physical activity during the postpartum period may also improve sleep (Ashrafinia et al, 2014; Vladutiu et al, 2014). Ahrafinia et al (2014) found a positive relationship between physical activity obtained during pilates, and sleep in postpartum mothers. Other research has been supportive, but less conclusive: Vladutiu et el (2014) suggested a positive relationship between physical activity and sleep; however, future research was recommended due to mixed findings. Physical activity could therefore be an important factor in interventions to improve key health outcomes of postpartum mothers.
Strengths
One of the key strengths of this study was the use of wrist-worn accelerometers over three assessment periods to assess physical activity and sleep simultaneously. This provided insight into key health behaviours at three time periods between 3–6 months postpartum. These data provided a more objective measure of physical activity and sleep, as the majority of research in postpartum mothers has used self-report assessments and/or been conducted with a cross-sectional approach (Wolfson et al, 2003; Wilkinson et al, 2004; Gunderson et al, 2008; Montgomery-Downs et al, 2010). Specifically, in terms of sleep, wrist-worn accelerometers have been validated against polysomnography, the criterion measure (de Souza et al, 2003; McCall and McCall, 2012; Kosmadopoulos et al, 2014). It has been suggested that wrist-worn accelerometry is an ideal sleep measure, as it is more accurate than self-report measures and does not require the invasiveness of polysomnography, which can lead to sleep disturbances (Morgenthaler et al, 2007).
Limitations
This study was not without limitations. First, although the use of accelerometers increased objectivity of the measures, issues with accelerometer placement may have hampered the accuracy of capturing activity. Similar to previous research, physical activity levels reported in this study were high and likely overestimated (Hildebrand et al, 2014). However, it is important to note that wrist-worn accelerometer outputs are correlated to energy expenditure as measured by indirect calorimetry, even in studies that found overestimation of physical activity (Swartz et al, 2000; Hildebrand et al, 2014). Wrist placement was used in the present study in an effort to increase compliance and deemed more practical for multi-day use (Hildebrand et al, 2014). Even though this may have skewed the data, physical activity levels were still assessed and compared between participants within this study, which maintained the validity of the findings. Secondly, due to the small sample size and limited data collection, it may be difficult to generalise the findings of this study. These findings may not represent all mothers or be indicative of physical activity and sleep trends at three time periods between 3–6 months postpartum, as data were collected for 4 days at three intervals with inconsistent amounts of time between them. Additionally, factors that may influence postpartum physical activity and sleep, such as other children in the home, amount of maternity leave from work, and history of physical activity and sleep were not gathered. Third, the sample was fairly homogenous: most of the participants were white, had at least some college education, and reported higher levels of income. More research is needed, with diverse populations of postpartum mothers to increase understanding of sleep and physical activity in all postpartum mothers. Furthermore, overweight/obese and normal-weight groups were not matched for demographics. Demographic differences, such as the percentages of mothers who work full-time, may influence findings and be responsible for some of the variance between groups. It is important for future research to continue to explore postpartum physical activity and sleep with a larger sample that can be based on demographic information. Lastly, other factors that could have directly impacted the study were not accounted for. For instance, mothers were not asked if they had any condition that might impact their sleep levels, such as insomnia, nor was the acute health of mothers recorded. Mothers who were swimming for less than 30-minutes may not have reported it as non-wear time, which would certainly have affected the total physical activity levels of those mothers, and the acute health of mothers was not considered, meaning that mothers also could have fallen ill during data collection, which, considering the small sample size, could have had a significant impact on the outcomes.
Conclusion
The present study examined postpartum mothers' physical activity and sleep while comparing physical activity and sleep of overweight/obese and normal-weight postpartum mothers. Overall, overweight/obese participants did not increase physical activity levels as much as normal-weight participants and all participants tended to lack sleep. Efforts to increase physical activity in postpartum mothers may be helpful in addressing both of these issues.

